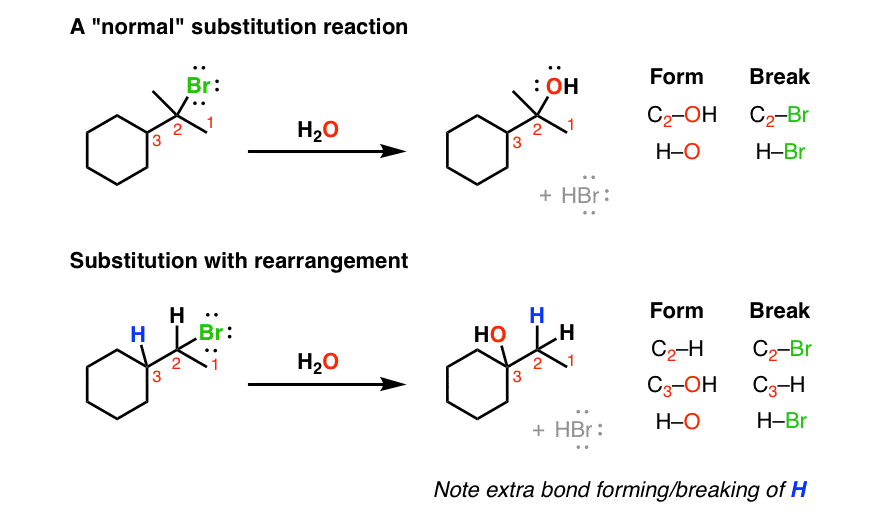
Draw the Carbocation Rearrangement Product of the Secondary Carbocation Shown.
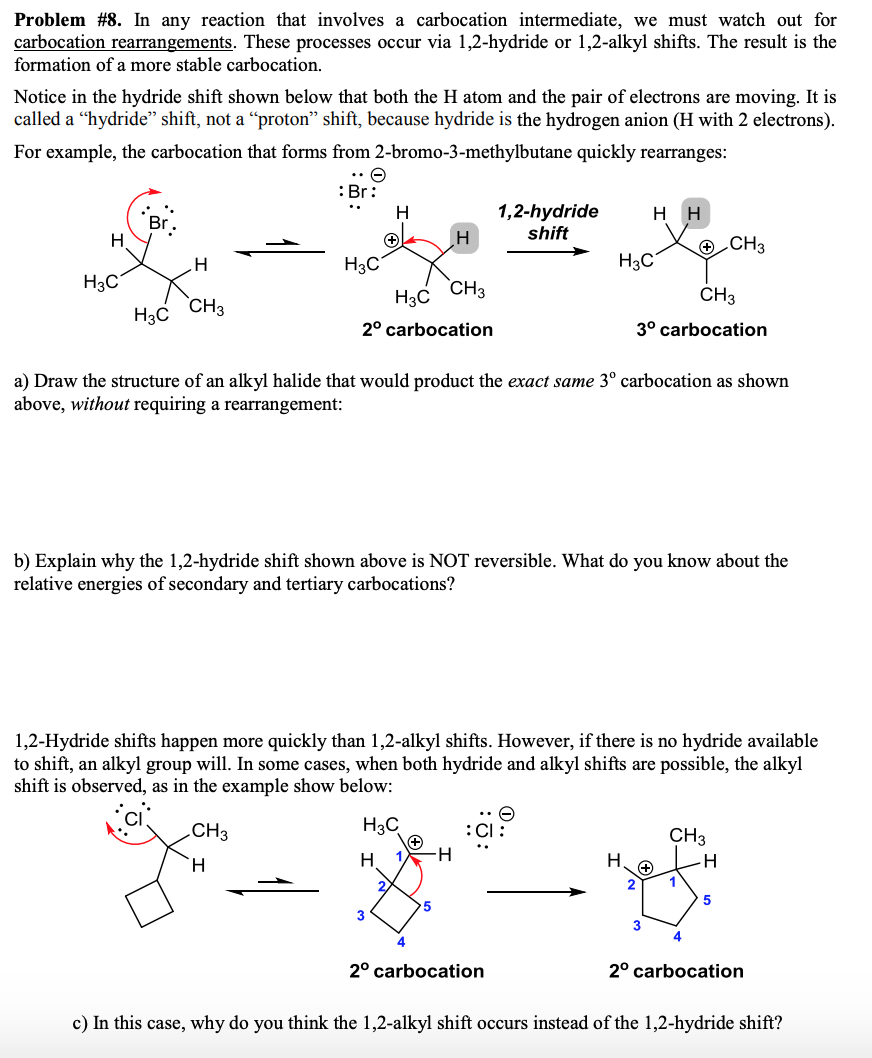
Formerly it was known as carbonium ion. If a secondary carbocation is vicinal to a tertiary carbon bearing a hydrogen a 12-hydride shift should occur.
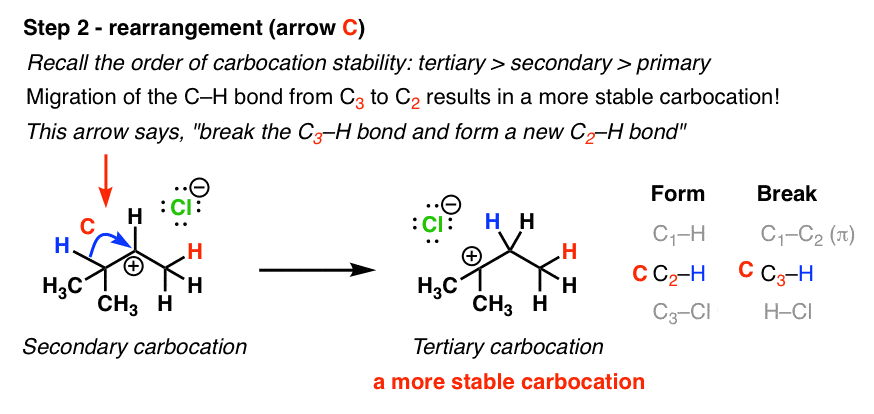
Rearrangement Reactions 1 Hydride Shifts Master Organic Chemistry
First week only 499.
. In this reaction the hydrogen atom on carbon 1 shifts to the positively-charged carbon carbon 2. And we know from the previous video that a tertiary carbocation is more stable than a secondary carbocation. In the above example you can see that we get a primary carbocation at first.
Carbocation stability - the same factors that stabilize carbocations are the same factors that stabilized radicals. 5 Draw the expected product of the given reaction. Draw the carbocation rearrangement product of the secondary carbocation shown.
Hyperconjugation polarizable bonds EDGs resonance. Step-by-step explanation It is known that alcohol undergoes dehydration if it is heated in the presence of acid. Now if you look at one of the carbon atoms next-door a hydrogen can migrate with its pair.
Alcohol A gives a reasonably stable 3 carbocation which reacts with the electron-rich benzene ring in a FriedelCrafts alkylation to give the product. However alcohol B gives a less stable 2 carbocation at least initially. Thats called a shift.
Carbocation Rearrangement occurs whenever the alcohols are converted into several carbocations and this phenomenon is termed as carbocation rearrangement. On the left is our alkyl halide ethanol is our solvent and on the right is our product. Start your trial now.
That is a plus one formal charge. The confusion may be due in part to the continued use of the terms edge-protonated and corner-protonated to describe PCP. Solution for Draw the carbocation rearrangement product of the secondary carbocation shown.
Draw the carbocation rearrangement product of the secondary carbocation shown. The first step should be loss of a leaving group. If a rearrangement is possible you should rearrange the molecule in order to obtain the major product.
View the full answer. The rate of this step and therefore the rate of the overall substitution reaction depends on the activation energy for the process in which the bond between the carbon and the leaving group breaks and a carbocation. Identify the type or carbocation formed and L whether a rearrangement will occur.
So lets look at this SN1 reaction. - Lecturer Since the SN1 mechanism involves the formation of a carbocation a rearrangement is possible. If a secondary carbocation is vicinal to a quaternary carbon a 12-alkyl shift should occur.
We know that the rate-limiting step of an S N 1 reaction is the first step formation of the this carbocation intermediate. Preparation and reactions of alcohols Alcohols can by prepared from alkyl halides epoxides alkenes etc. So this is the rearrangement that we.
Now lets look at this resulting carbocation. This is known as a hydride-shift. When treated with acid mathrmC-mathrmOH bond is cleaved heterolytically to form a carbocation which.
Select Draw Rings More Erase. The carbon in red is this carbon and thats a secondary carbocation because the carbon in red is directly bonded to two other carbons which I just marked there in magenta. And let me draw in the plus one formal charge on the carbon in red.
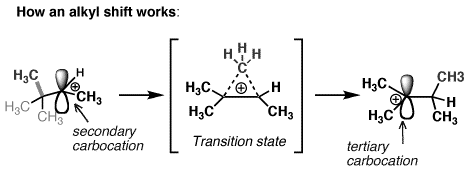
H i Strategy. Identify the type 0f reaction that Will OCCUR The carbocution formed after protenation of the alkene is. It turns out that carbocations are going to be able to rearrange to more stable positions if theyre adjacent to the carbocation and if it has more R groups than the carbocation has at the moment.
When are rearrangements possible. In a rearrangement a group moves from one atom to another in the same molecule. So this is a secondary carbocation.
Stability of carbocation intermediates. We can basically say that they are carbon cations. Hence it is a 12-shift.
And theres a few different ways that this can happen. Most are migrations between adjacent atoms and are called 12-shiftsCarbocation rearrangements occur more frequently on secondary carbocations to form tertiary which are more stable and energetically more favorableIn general the bonding electrons of a. The carbon thats in blue is directly bonded to one two three other carbons So this is a tertiary carbocation.
A rearrangement will occur in the carbocation intermediate shown. Carbocation rearrangement can be carried out to a reaction that does not. Draw a curved arrow to show the rearrangement and draw the carbocation product.
Carbocation rearrangements are common in organic chemistry and are defined as the movement of a carbocation from an unstable state to a more stable state through the use of various structural reorganizational shifts within the molecule. Modeling of product distributions in biomasspetroleum refining5 This paper clarifies the role and structures of PCP in carbocation rearrangement supported by a large body of new computational results. Next we think about the possibility of a rearrangement.
This is the best answer based on feedback and ratings. A hydrogen can move from one atom to the atom next door if it will increase the stability of the carbocation 3 0 2 0 1 0. The rearrangement of the secondary carbocation to more stable tertiary carbocation is shown in explanation part.
A carbocation is a molecule in which a carbon atom has a positive charge and three bonds. Carbocation today is defined as any even-electron cation that possesses a significant positive charge on the carbon atom. Carbocation - sp2 hybridized trigonal planar empty unhybridized p-orbital that juts out of hybridized bonding plane and formal positive charge.
Carbocation rearrangements Organic Chemistry 1. Professor Davis shows several examples of how secondary carbocations can rearrange themselves by hydride shifts alkyl shifts and ring expansions to form mor. However if a hydrogen moves from the secondary carbon to the primary we can get a secondary carbocation.
So basically what your criteria is is this. In simple carbocation comprises ve charge in a molecule that is connected to 3 more groups and holds a sextet. As carbon 1 now only has three bonds it has gained a.
Determine the regiochemistry of the addition reaction. A 12-hydride shift is a rearrangement reaction that can occur if there is a hydrogen atom on the carbon adjacent to the positively-charged carbon of a carbocation.

Solved Problem 8 In Any Reaction That Involves A Chegg Com
Carbocation Rearrangement Definition Methodology Alkyl Shift

Rearrangement Reactions With Practice Problems Chemistry Steps

Sn1 Mechanism The Nucleophile Attacks From Both Sides Carbocation Giving A Racemic Mixture Organic Chemistry Chemistry Lessons Chemistry Worksheets
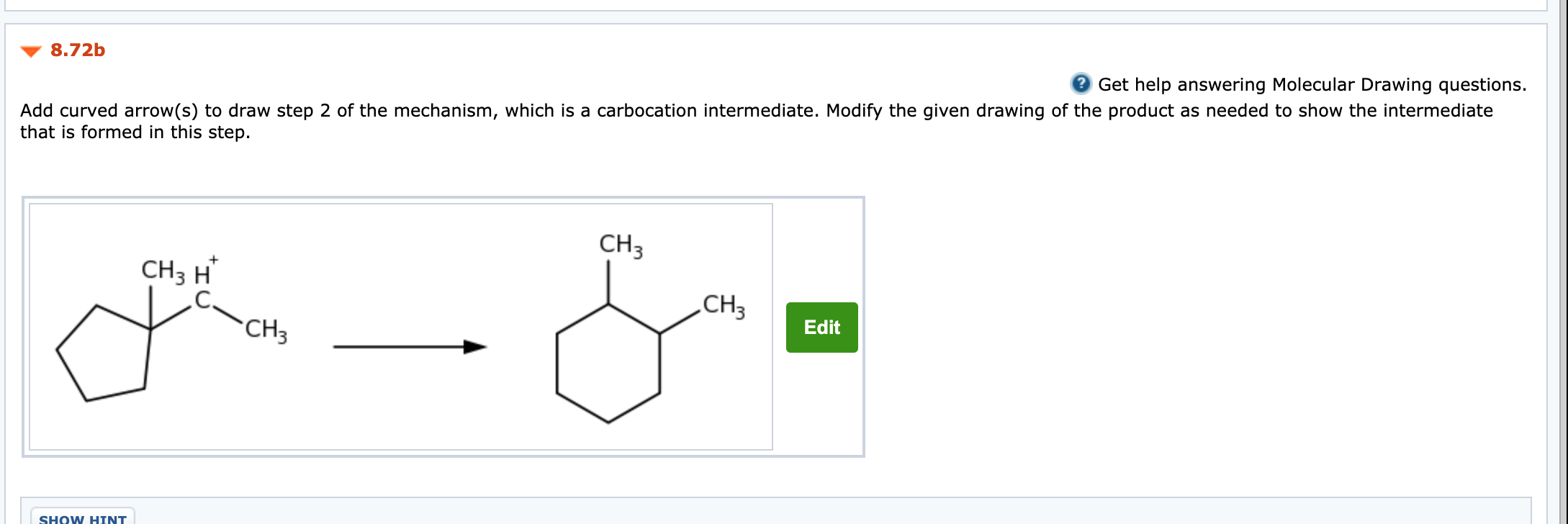
Solved Practice Problem 08 72 The Accepted Mechanism For The Chegg Com
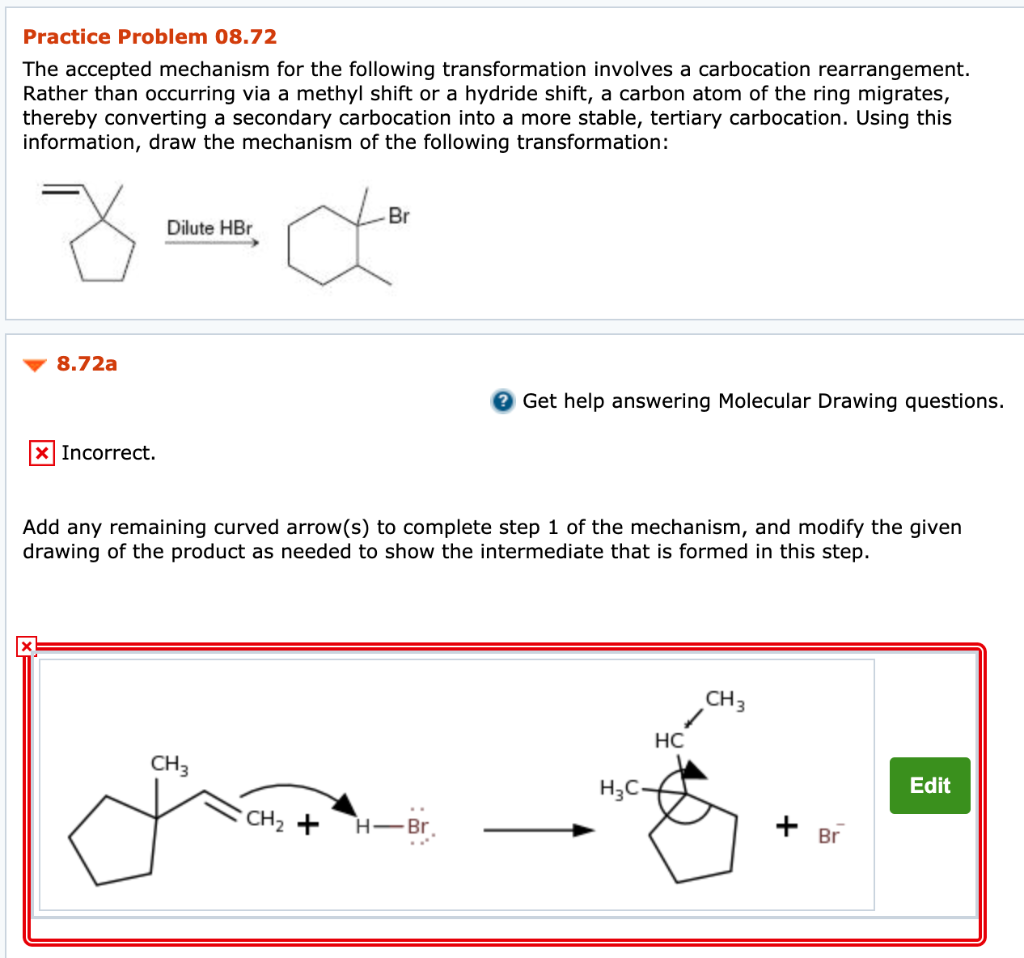
Solved Practice Problem 08 72 The Accepted Mechanism For The Chegg Com

Rearrangement Reactions With Practice Problems Chemistry Steps
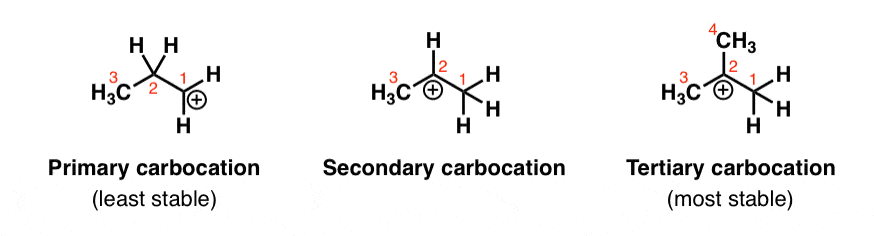
Rearrangement Reactions 1 Hydride Shifts Master Organic Chemistry
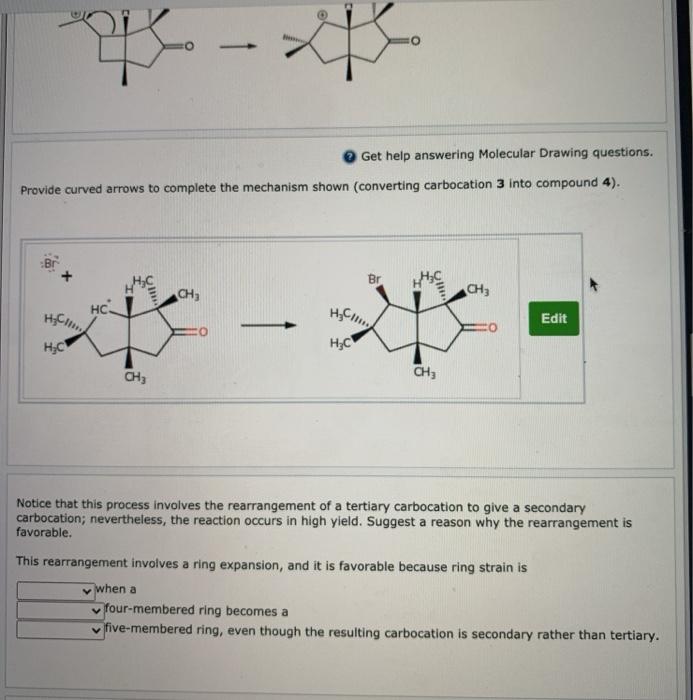
Solved Carbocation Rearrangements During Hydrohalogenation Chegg Com
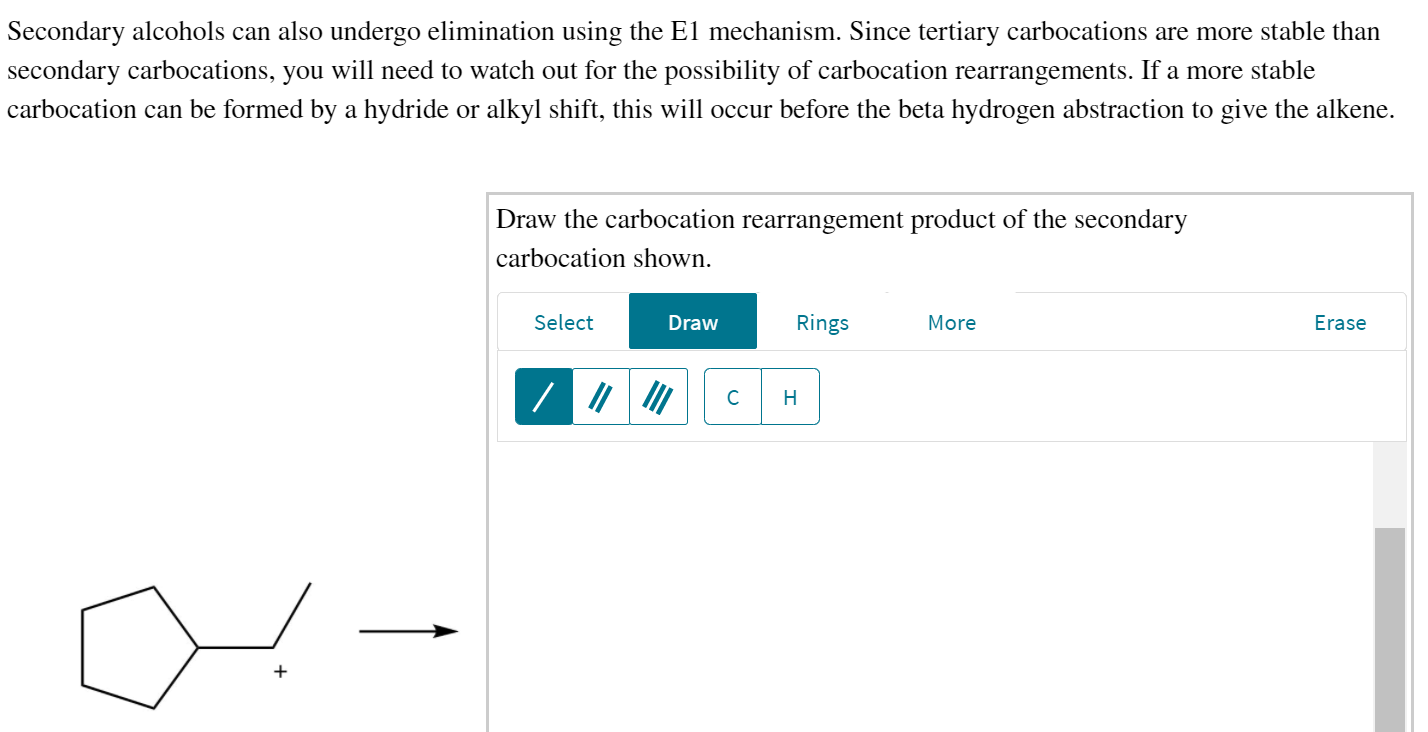
Solved Secondary Alcohols Can Also Undergo Elimination Using Chegg Com

E1 Energy Diagram Transition State Forming A Double Bond Reactions Energy Activities Chemistry
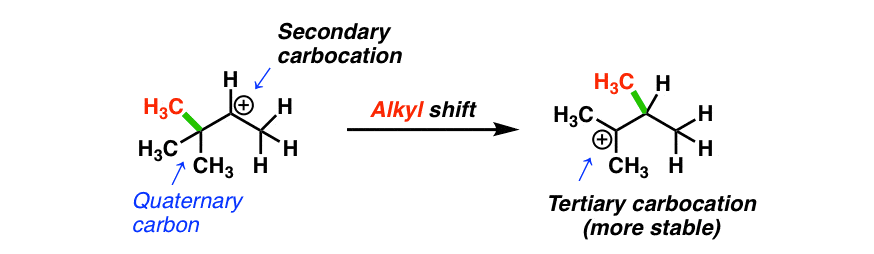
Rearrangements Alkyl Shifts And Ring Expansion Reactions
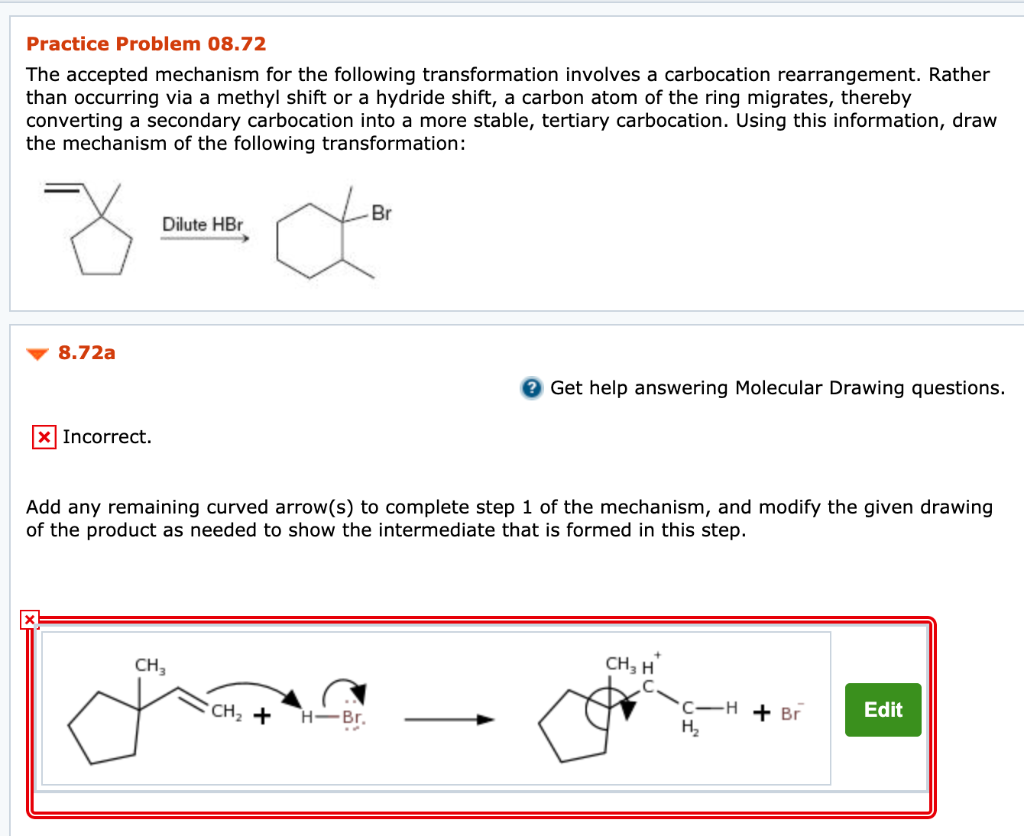
Solved Practice Problem 08 72 The Accepted Mechanism For The Chegg Com
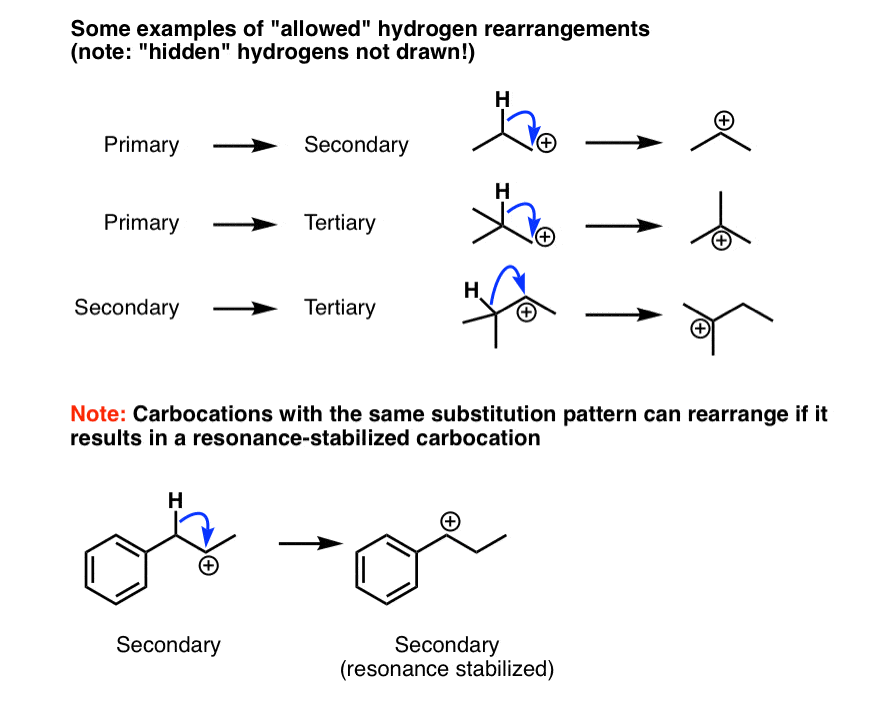
Rearrangement Reactions 1 Hydride Shifts Master Organic Chemistry

The E1 And Sn1 Reactions Always Compete Since Both Form The Same Carbocation Organic Chemistry Reactions Chemistry

3 Factors That Stabilize Carbocations Master Organic Chemistry Organic Chemistry Chemistry Lessons Organic Chemistry Study
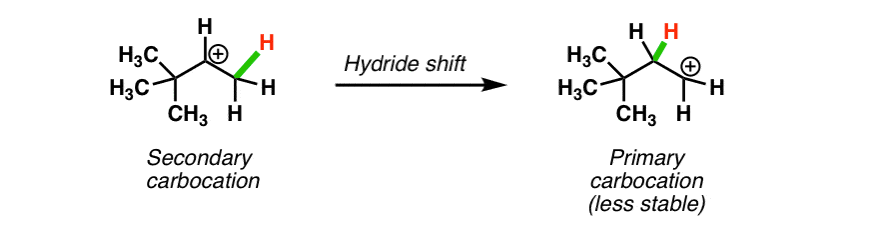
Rearrangements Alkyl Shifts And Ring Expansion Reactions
Rearrangements In Alkene Addition Reactions Master Organic Chemistry

Comments
Post a Comment